i NEWS PAK-CHINA
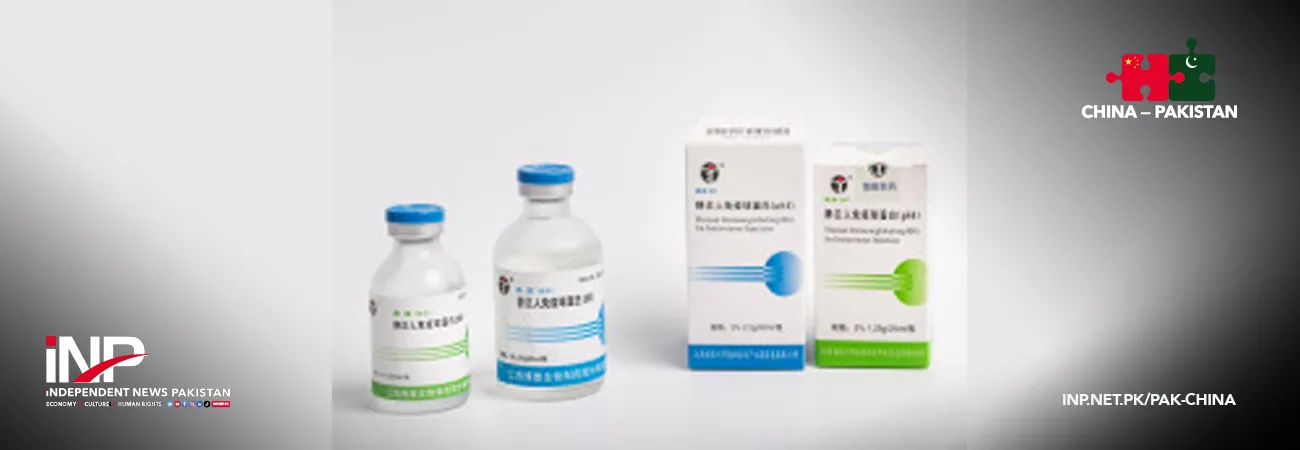
Guangdong Shuanglin Bio-pharmacy Co., Ltd., a subsidiary of PLBIO, has secured regulatory approval from Pakistan for its Intravenous Immunoglobulin (pH4), a key blood-derived therapeutic product widely used in clinical practice, its parent company announced in response to investor inquiries.
According to a report carried by China Economic Net (CEN) on Thursday, the drug registration certificate was jointly issued by Pakistan's Ministry of National Health Services, Regulations, and Coordination and the Drug Regulatory Authority of Pakistan.
This approval enables expanded access in Pakistan to advanced biotherapeutic options, particularly for treating immune-related disorders.
The company has entered into an Exclusive Licensing and Supply Agreement with local distributor 3A Diagnostics, facilitating the product's market entry.
It noted that while the product is now available locally, no plasmapheresis centers have been established in Pakistan.
Intravenous Immunoglobulin (pH4) is a sterile, purified preparation derived from pooled human plasma, primarily containing IgG antibodies.
It plays a vital role in replacing deficient antibodies in patients with immune disorders and in modulating abnormal immune responses in autoimmune conditions.
The introduction of this WHO-standard product strengthens Pakistan's clinical capacity to manage conditions such as primary immunodeficiencies, autoimmune diseases, and severe infections.
The partnership also supports the resilience of Pakistan's pharmaceutical supply chain and aligns with efforts to diversify sources of essential medicines, contributing to public health infrastructure and bilateral healthcare cooperation between China and Pakistan.
Credit: Independent News Pakistan (INP) — Pak-China